Surfaces of materials are the first line of defence against environmental attack and provide the opportunity to implement enhanced functionalities to structures and products. The binding theme of the Cluster 3 ‘Durable Surfaces’ is the fundamental understanding of surfaces, interfaces, and interactions with the environment. The projects include in-depth surface science and engineering investigations to both (electro)chemical and physical phenomena, as well as advanced experimental and theoretical modelling approaches in these areas.
Local ratiometric pH sensing at the surface of an electrocatalyst (TRANSCRIPT)
Transforming waste gases of steel making operations into valuable products through both thermo- and electrocatalytic processes, involves design and optimization challenges of both catalysts and reaction cells. In that sense, developing a set of local sensing tools is essential, so that local pH, temperature and reagent concentration near the reaction surface can be monitored, reaction pathways and mechanisms of CO2 reduction can be revealed and the activity and selectivity of catalysts can be fine-tuned.
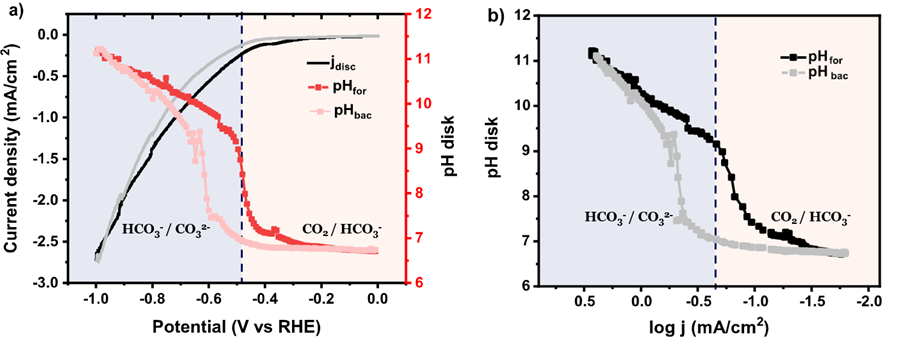
Figure: (a) Variation of the current density and interfacial pH recorded during cyclic voltammetry in CO2-saturated 0.1 M NaHCO3, b) interfacial pH as function of the logarithm of the current density during the cyclic voltammetry from a).
Using a rotating ring-disk electrode (RRDE) as a real-time pH and CO2 sensor, successful measurements of the interfacial pH as well as faradaic efficiency during the carbon dioxide reduction reaction (CO2RR) were performed, making it possible to unravel the influence of interfacial environment on the competition between CO2RR and the hydrogen evolution reaction. This research signifies the remarkable difference between interfacial and bulk parameters. The interfacial environment in acidic media can be highly alkaline even with a strong buffer (e.g., phosphate) under forced convection. The RRDE sensor used here allows to trace the change of competition between multiple reactions with the evolving interfacial environment during reactions, which might be a promising alternative for studies in other electrochemical systems.
Advanced Measuring and Modelling of Thin-film Corrosion (ThinCorr)
Atmospheric corrosion takes place under a thin layer of electrolyte or tiny droplets that form by condensation or absorption of water. Atmospheric corrosion rates in practice are reported significantly larger as compared to thick film or bulk electrolyte conditions, a.o. owing to a greater availability of oxygen. A challenge in this regard is to conduct electrochemical evaluations under tiny droplets or thin layers of electrolytes. Last year, a new approach was proposed to numerically predict and study atmospheric corrosion for ranging droplet size distributions and the influence of the droplet geometry.
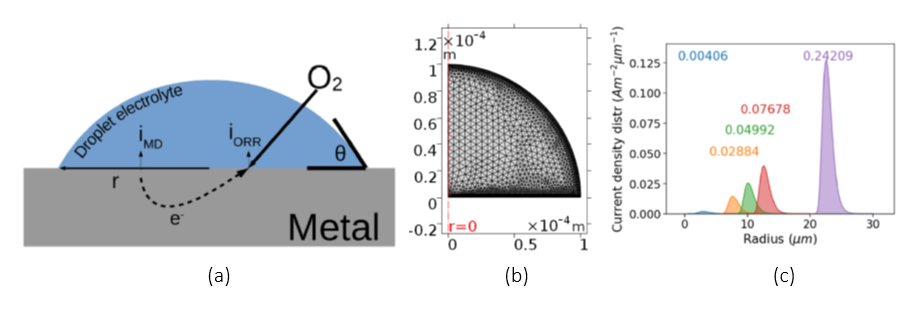
Figure: (a) Illustration of the oxygen reduction and metal dissolution current densities in a droplet, (b) the mesh for a single droplet simulation and (c) droplet corrosion current distributions for different droplet dimensions on iron.
The proposed methodology allows for a corrosion prediction based on observed droplet size distributions and droplet contact angles. A mechanistic finite element model, including oxygen transport and Butler-Volmer kinetics, is solved in order to obtain the current density as a function of the droplet geometry. This is done for a range of both droplet radii and contact angles. The computed corrosion current densities are then used as input for imposed droplet size distributions. This allows for a calculated material loss estimation for different distributions and electrolyte configurations and shows the extent of the impact of the droplet size distribution on atmospheric corrosion. Atmospheric corrosion in different environmental conditions can now be evaluated by regulating the electrolyte height, droplet size and distribution as well as solution chemistry of the droplets.
More information
For more information about our Durable Surfaces Cluster, please contact the Yulia Fischer.